Making chlorates - gouging rods impregnated in paraffin
preparation of anodes
5 gouging rods (8mm in diameter and 305mm in length) were weighted, one having 26g and 5 125.5g


getting rid of copper layer
Since copper would easily corrode away and contaminate the product, it has to be stripped away, this also allows to impregnate the graphite.
Rods were wrapped in a wire connecting them to 5v psu as anodes and some old rod (it can be metal) as cathode were placed in water bottle.

Then the bottle was filled with water and some electrolyte. I've used dirty sulfuric acid which has high conductivity even at very low concentrations, but even table salt will do.
The current reached 6.1A and water turned blue pretty quickly due to copper sulfate formation.

Halfway the connection was changed to the other side as not everything was below water level.
In about an hour rods were copper free, they were pretty clean at 40 minutes but i didn't want to scrape the copper from the sides so they ran longer. Also the current was too high for them and caused the outside of the rods to become a little spongy making them good at painting hands.
Note that the very ends of the rods had some copper residue but it doesn't matter as they will be electroplated in the next step.

electroplating copper
I will be using multiple anodes side by side with limited current meaning that if connection to any of them breaks the current to others will increase and will erode them quickly.
Making a good connection to graphite is not easy with crocodile clips, aluminium or tape. These types of connections tend to easily disconnect by themselves or by movement of rods, they also often heat up.
The best way to ensure good connection is to electroplate copper (the easiest metal to electroplate) and connect to it.
Somewhat concentrated solution of copper sulfate and ethanol was put into narrow container (yogurt cup).

Water level is important as it will be the size of copper layer.

Then delaminated copper wire is made into a loop and put into the cup as the anode.

A rod is placed into the solution, secured with crocodile clips acting as cathode.
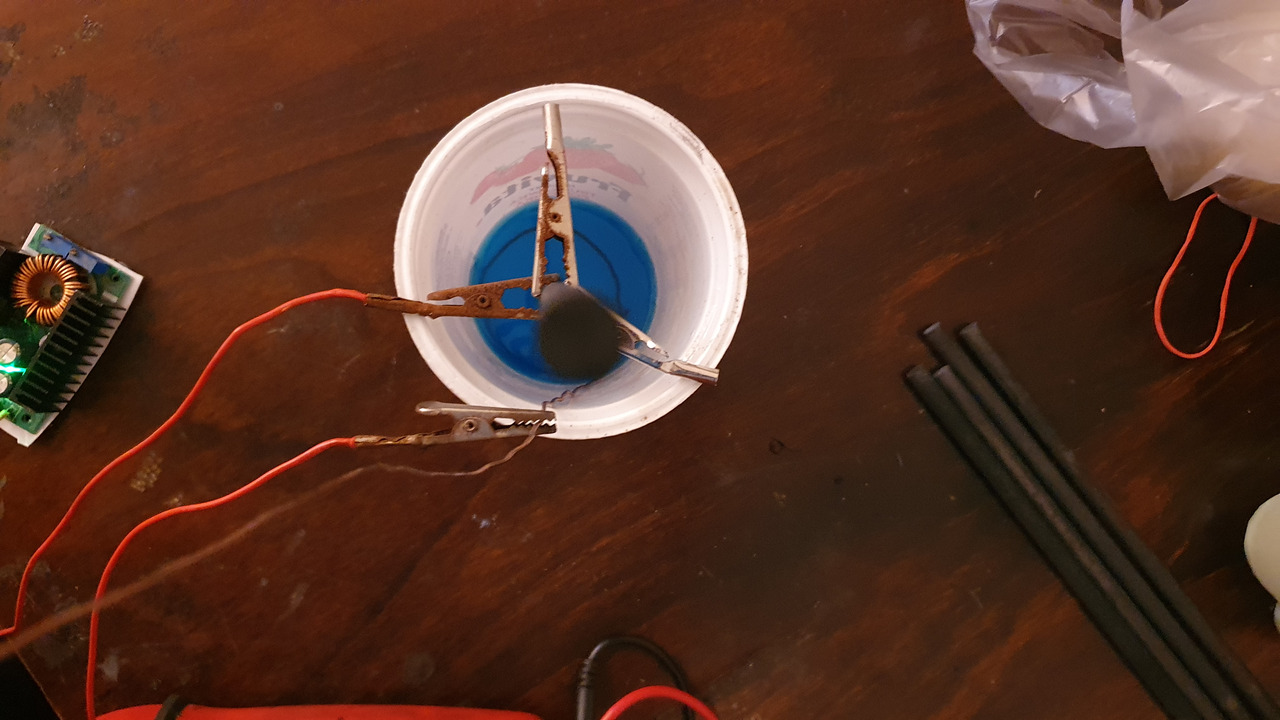
The current has to be limited in this step, i've used some buck converter.
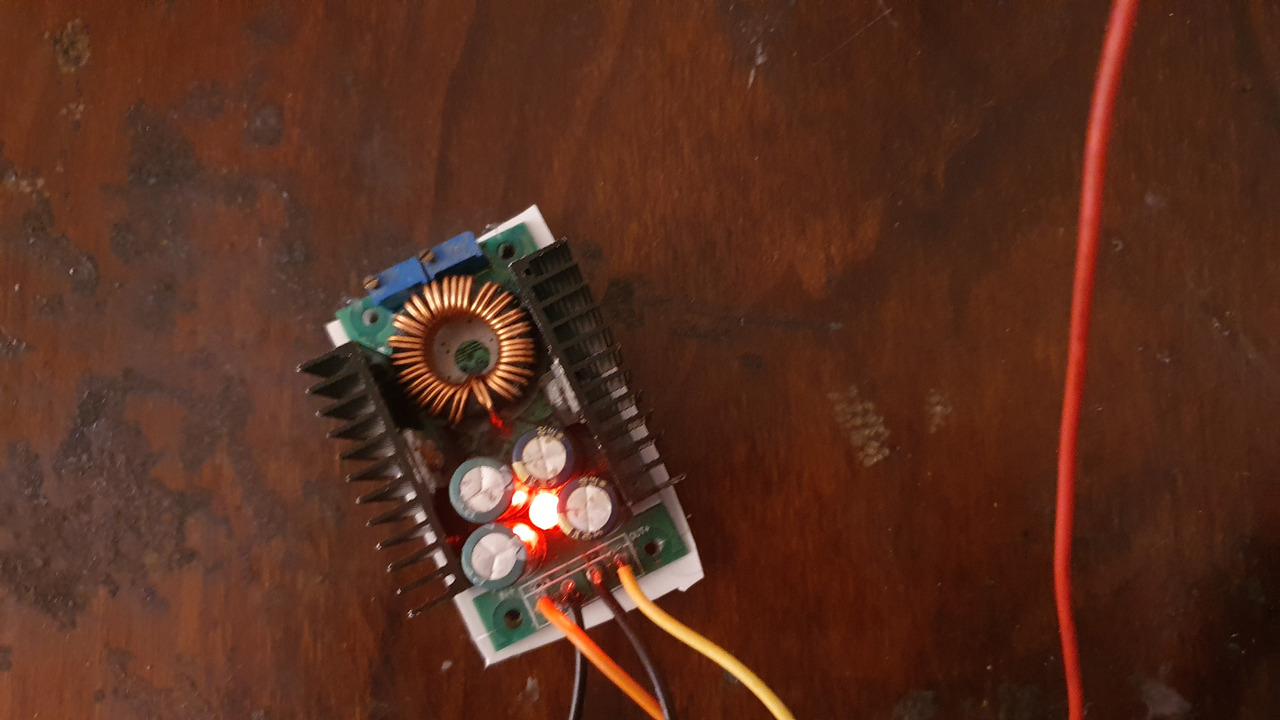
At first i used 0.4A but it caused a spongy layer so i've rubbed it of with paper and set the current to 0.2A. This has worked for the rest of them.

Rods were also cleaned with paper 2 times when running to ensure that no sponge has formed.
Each one took around 1.5 hour. At the third rod the copper loop had to be replaced as it was eaten away.
After this step they have weighted 23.1g for single rod and 116.6g for 5. The weight has dropped by 2.9g for single rod and 8.9g for 5. Note that every time a single rod is weighted it is chosen randomly, apparently i had chosen some exceptionally light rod for this test.


impregnation
Gouging rods have a spongy structure which allows the water to seep in making the electrolysis happen inside the rods. Gases produced in it cause pressure on brittle graphite which erodes the rods.
linseed oil
I previously made chlorate cells with unimpregnated rods and impregnated with linseed oil. The unimpregnated rods completely eroded leaving short pieces of graphite at the end. While linseed oil rods survived leaving some degradation in the middle (new rod for comparison).

They were soaked in copper pipe with soldered end for 3 days each and were dried for a week on radiator (this was done in winter).

After weighting and comparing it to before they had gained 0.5g each which was little. They should have been put into a vacuum chamber (that i don't have), it would also make soaking it pretty instantaneous. But even with this low amount the erosion rate noticeably decreased.
paraffin
In this run i will try impregnating them with paraffin.
I've welded a pipe to small plate making a narrow waterproof container.
Then i have searched my house for candles. I found 1 scented and 2 unscented dyed red.
I've put the wax of scented candle into a pot and put it on my rocket stove to evaporate the light oil and scented solvent in it. When it started to smoke excessively i've put the 2 dyed candles.
They have melted pretty instantaneously and started smoking like a smoke grenade. After 10 seconds it lit on fire, the flame was leaving a lot of coal residue.
As it turns out the boiling point of paraffin is above 360C but before it reaches it, it generates huge amounts of smoke and at around 220C the flash point is reached so the smoke lits on fire.
I have covered the pot with some metal plate and it put out the fire. I took the pot out of the stove and uncovered it, which again lit on fire. I covered it again and preheated the pipe. Then i put it into a small hole in the ground so it would not tip over.
After a minute i've started pouring the paraffin into the pipe and it began to boil spilling a little of paraffin.
I've added the first rod and it again began to boil or at least released a lot of air bubbles, some of paraffin has spilled, i've put the rest of the rods attaching crocodile clips to them so they would not fall in.

A lot of bubbles were released for around 15 seconds.
Then i've waited for paraffin to cool down just until it starts to solidify. While waiting i've mixed rods around for better soaking. If paraffin sets completely it will be hard to pull the rods out.


Then rods have been well scraped with a knife.

Weighting the results single rod had 24.2g and 5 had 122.9g. This is overall 6.3g increase in weight, also i think that i've lost a gram of graphite due to my scraping.


finishing
Rods were soldered to short cables cut off from pc psu and connection was secured with shrink wrap.

preparation of cathodes
Two rectangles 37cm by 15.5cm were cut from 0.3mm thick metal sheet. From them two rectangles 7cm by 8.5cm starting from the end were cut off leaving a 7cm squares at the ends that will be used for mounting. Then they were polished with angle grinder and area below rectangles was perforated with 10mm drill. Perforation will reduce concentration differences in the solution.
frame for electrodes
In a thick piece of plank 5 8mm holes were drilled at the top. Then 2 parallel lines 3cm from the holes were marked. Using a hacksaw 9cm notches along the lines were cut.
Anodes were mounted in the holes and cathodes were put in the notches.
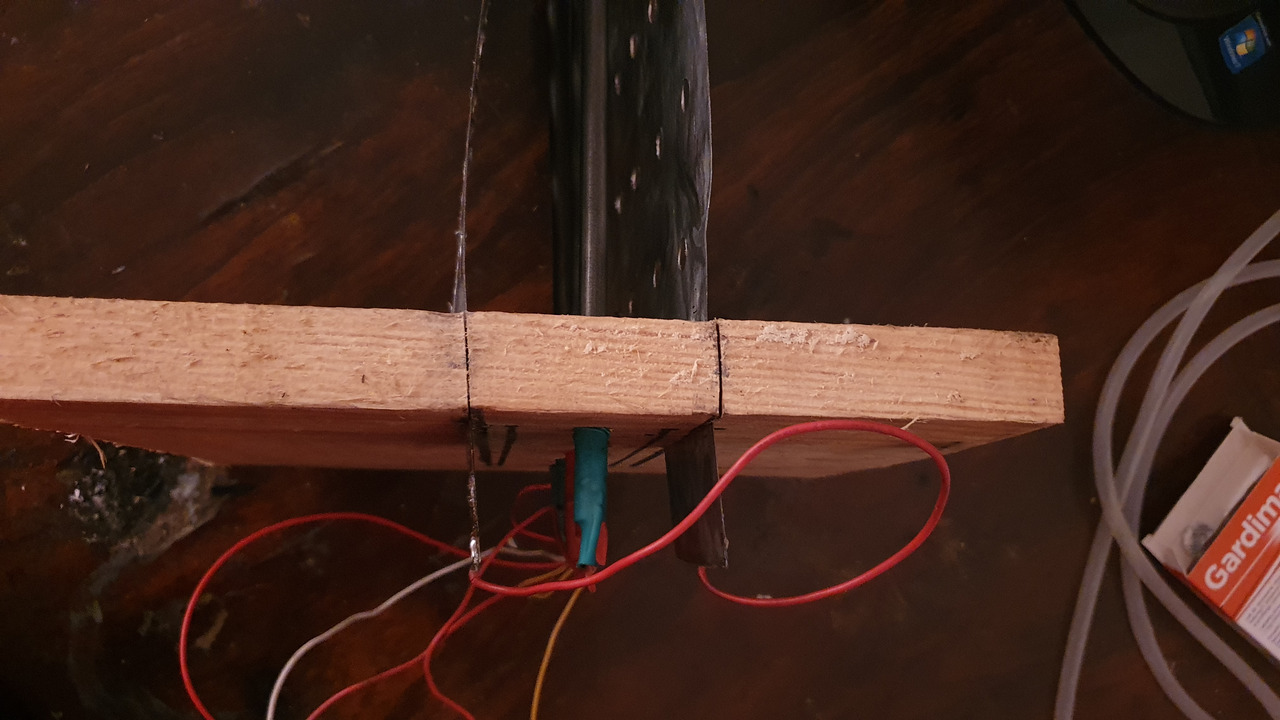
Cathodes were bending away from the anodes so they were tied together with a piece of wire. Plastic spacers were also glued to one side to prevent electrodes from touching.

Cathodes were connected with a wire soldered to them. Cables of the anodes were soldered to one point.
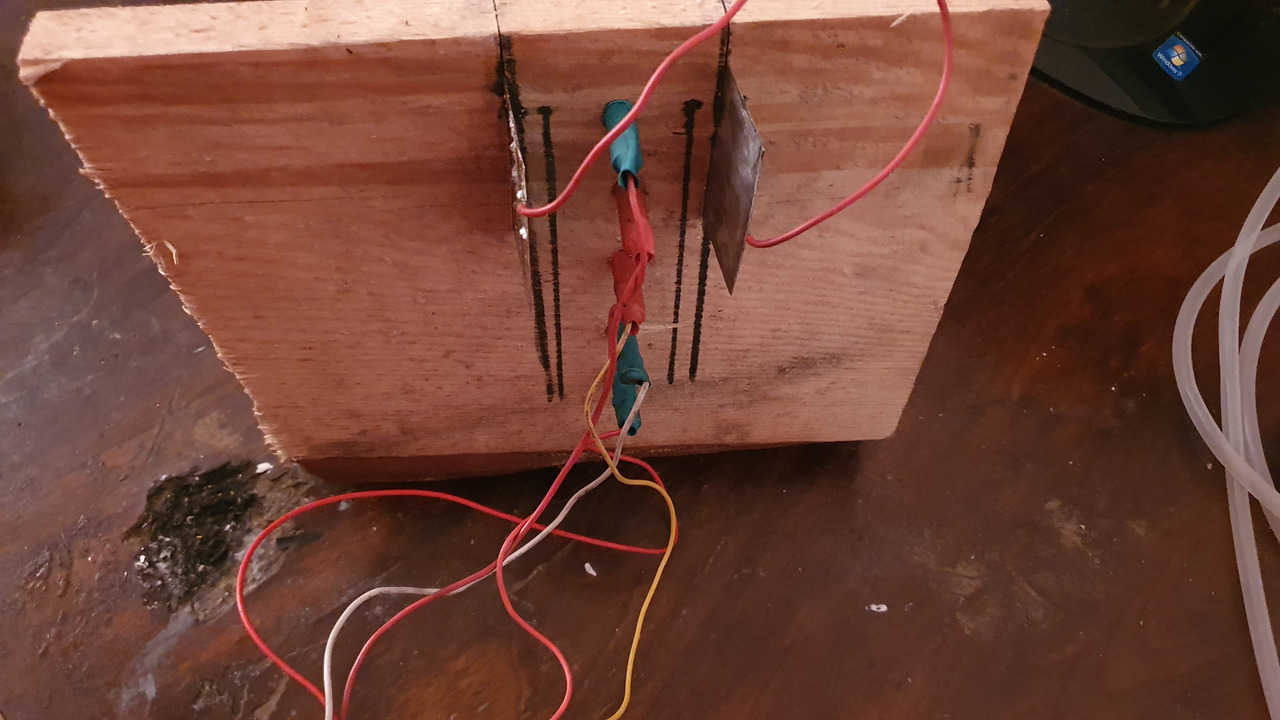
container
I've cut off the top of 5L dish soap container and marked a line at 17cm.


Then i've filled it with water to marked level. Water weighted 3850g at 23C. This equates to 3850/0.997541 = 3859.5cm^3 of volume.
5 rods submerged to 17 cm have volume of 5 * 17 * 0.4 * 2 * 3.14159 = 213.6cm\^3. And two cathodes 0.3mm thick and 15.5cm wide have 2 * 17 * 15.5 * 0.03 = 15.8cm\^3.
So the water volume with the electrodes added will be 3859 - 213.6 - 15.8 = 3.6296L.
current density
Graphite anodes have to be run at current densities of around 0.03mA/cm^2 or 0.04mA/cm^2 if they are impregnated and good temperature and pH control is taken. I will run it at 0.029 mA/cm^2. Shortening the length of it to water level and using 5 of them the current should be 0.8*3.14 * 17 * 5 * 0.029 = 6.192A.
psu
I've used a 19V laptop psu connected to a buck converter to limit current.
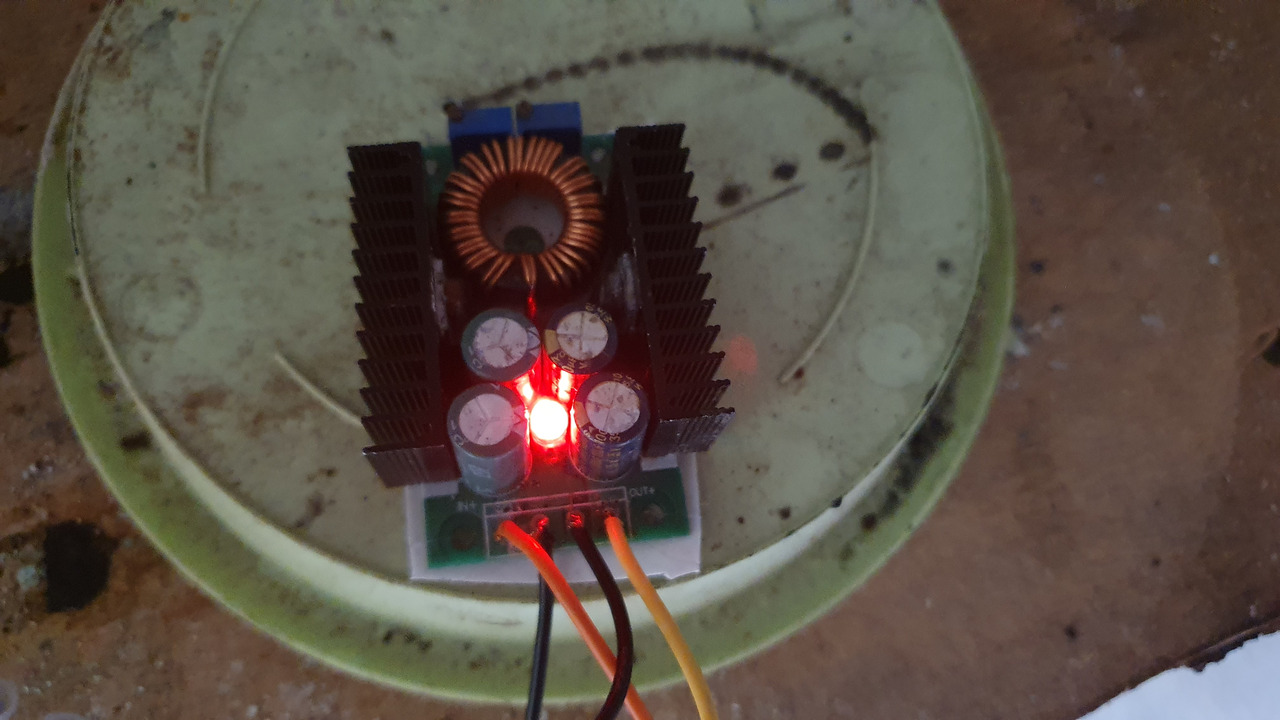
pH control
Chlorine gas produced can escape before it reacts with sodium hydroxide increasing pH of the solution. Graphite erodes much quicker in basic conditions. High pH also reduces efficiency to about 50%-60%.
pH can be controlled by periodically adding very dilute hydrochloric acid e.g. 0.2% drop wise every 5 minutes. Rate of addition should depend on pH of the cell just to keep it neutral. If the electrolyte is very acidic cell will be producing chlorine gas.
Another method is to have some chloride salt that has insoluble hydroxide dissolved in solution making a pH buffer. Salt either reacts with sodium hydroxide and creates sodium chloride and insoluble hydroxide or gets oxidised by the electrodes coating the cathode in conductive layer of hydroxide. This is beneficial as it makes cathode more likely to reduce water rather then chlorate increasing current efficiency. Suitable salts for pH buffer are calcium chloride and magnesium chloride.
pH control can increase current efficiency above 80%.
graphite making chlorine
Graphite anodes should be run at low temperatures to avoid erosion. They also have to be run at low current densities so temperature cannot be high anyway. But higher temperature increases current efficiency as chlorine reacts faster with sodium hydroxide instead of escaping.
Graphite anodes generate a lot of chlorine gas, especially at the beginning of the run. They should never be run at home, as in a couple of minutes your room will reek of chlorine, after half an hour of them running you will have trouble breathing.
This also means that they are not perfect for pH buffers because more chlorine escapes and a lot of hydroxide precipitate will be formed, it's still better then no pH control though.
electrolyte
To ensure that i'm using concentrated salt solution an excess of sodium chloride with water is added to a bottle. As long as salt is visible at the bottom the solution is concentrated.
The sodium chloride solution at 26C filling container to the level weighted 4689g. Sodium chloride at this temperature has solubility of around 360g/L.
So concentration of salt is 360g / (360g + (1000ml * 0.996786g/L)) = 360g / 1356.786g = 0.2653329
This solution has 0.2653329 * 4689 = 1244.14g of sodium chloride.
However it's crucial to leave 100g/L of sodium chloride in the solution at the end of run as below that any anode gets rapidly destroyed. You can easily judge if cell needs to stop running by anode eroding.
So from this solution salt that has to be left is subtracted leaving 1244.14 - (3.6296*100) = 881.18g of salt to be converted.
calculating time to completion
To oxidize one mole of sodium chloride into a mole of sodium hypochloride 2 moles of electrons are needed, 3 moles of sodium hypochloride decompose to one of sodium chlorate and 2 of chloride. This means that to convert one mole of sodium chloride to chlorate 6 moles of electrons are needed.
q / F = mol
q = mol * F
(I * t) / I = (mol * F) / I
t = (mol * F) / I
Applying the above to simplified reaction
t = (m * (1/58.5) * 6F) / I
t = m * ((1 / 58.45) * 578911.99) / I
It has to be noted that current efficiency is not perfect. I have pH control so i very optimistically assume 80% efficiency.
t = m * (1/current efficiency) * ((1 / 58.45) * 578911.99) / I
In this equation m is the mass of salt. 58.45 is the molar mass of sodium chloride, if you are using potassium chloride replace it with 74.45.
So currently the cell has to run (881.18 * (1/0.8) * ((1 / 58.45) * 578911.99) / 6) = 1818240s = 505.06hours = 21.04 days. And should amount to (881.18g / 58.45) * (58.45 + 3*16) = 1604.81g of sodium chlorate.
cell running
2024-08-02 15:15
5ml of conc. hydrochloric acid and 30g of hydrated magnesium chloride were added to the solution


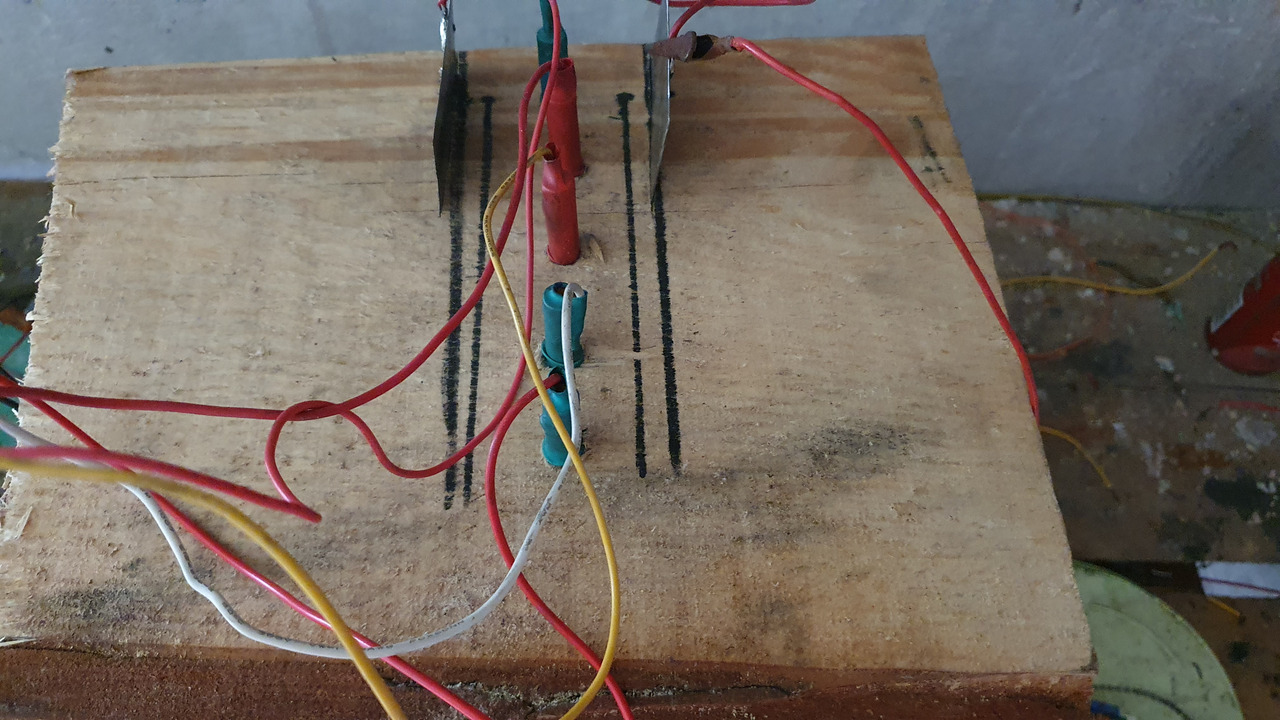
Solution quickly started to take on a yellow hue indicating formation of sodium hypochloride. It also started to produce huge amounts of chlorine gas to the point that to make adjustments to the cell i had to come out of the room around 15 times just to take a breath.
My buck converter steadily dropped current from 6.2A to 5.8A-6A so it's hard to predict the end of the run.
2024-08-05 11:39
electrolyte was basic so another 20g of hydrated magnesium chloride was added making the pH neutral. Smell of chlorine in air considerably decreased.
Temperature of cell settled at 34C.

2024-08-06 15:20
10g of hydrated magnesium chloride was added

2024-08-08 10:50
100g of hydrated magnesium chloride was added and 271g of sodium chloride solution. 271*0.2653329 = 71.9g


2024-08-13
20g of hydrated magnesium chloride was added and 230.4g of salt solution = 61.13g. Smell of chlorine in air became tolerable.
2024-08-17
40g of hydrated magnesium chloride was added and 342.5g of salt solution = 90.87g
2024-08-20
20g of hydrated magnesium chloride was added and 206.6g of salt solution = 54.81g
2024-08-24
40g of hydrated magnesium chloride was added and 385g of salt solution = 102.15g
Anodes started to visibly erode

Everything above water level became crusted in salt, salt crust even formed a layer floating on water. Although from the top cell water looks murky and dark it's actually still transparent.

2024-08-29
80g of hydrated magnesium chloride was added
2024-08-31
12g of hydrated magnesium chloride was added and 282.2g of salt solution = 74.87g. The current has drifted to 5.71A.
2024-09-04
228g of salt solution was added = 60.49g
2024-09-05 17:11
End of the run






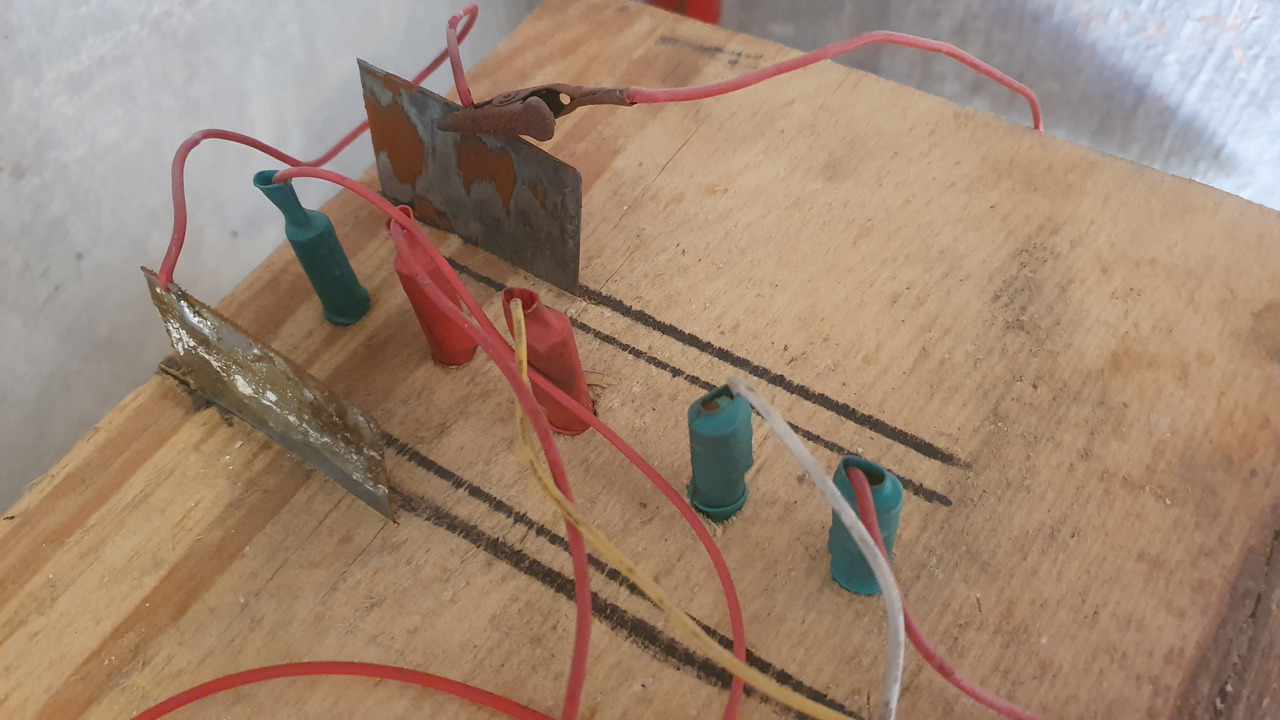






In total cell run for 34 days, 1 hour and 56 minutes.
372g of hydrated magnesium chloride was added, which is (372/(235.45+24.31+618))(235.45+24.31) = 174.293g of magnesium chloride.
1760.36g of salt overall added from which 1397.4g of salt could be converted to (1397.4g / 58.45) * (58.45 + 3*16) = 2544.96g of sodium chlorate. (1397.4 * (1/0.8) * ((1 / 58.45) * 578911.99) / 6) = 2883417s = 800.94hours = 33.372days, cell was run longer than that because current decreased with time, i probably stopped it too early.
I've tried to measure ph of the solution to ensure that no magnesium chloride is left, but ph paper quickly fades from green to brown color, probably gets bleached. In my previous run's it would get bleached white after a couple of hours.

filtering
The solution is full of graphite particles and magnesium hydroxide sludge. I've left it for 2 weeks and half of it became clear.


I've decanted it into another cleaned 5l container.


It was more yellow then on picture, and smelled a bit like mushrooms and chlorine.

The left over sludge was washed with a bit of water and many attempts were made to filter it. Usually i've filled a funnel once and when most of it separated i've filled it again, but sludge has settled to the bottom blocking any flow. Then i'd take some rod and try to stir it, breaking through the filter.



The resulting liquid weighted 5600-174 = 5426, with a bit still filtering.

after a month
For various reasons filtering took me more than a month.




I've measured with a caliper the loss in diameter. Most of the rods are above 7.4mm, tips have smaller diameter, the smallest one is 6.4mm. Anodes seem to be in good condition and probably could survive another run, although another soaking in paraffin would be necessary as it didn't penetrate them much.
Overall, paraffin is the best impregnation yet.
trying to make a quick demonstration
I've wanted to purify small amount of chlorate and show it burning with sugar.

I've quickly learned that i didn't clean the container completely from soap as the thing boiled over.

When it settled, a vivid yellow solution was seen (more so than on image).

After boiling half of it, there was already some precipitation.

Left over solution after filtering.

The precipitate was washed but it did not dry. I've made 3 attempts at mixing it with sugar and lighting it and all of them failed. First one was wet, second small amount torched, and the third drying on electric stove this failed because of not being powdered enough.
In conclusion sodium chlorate is bad for pyrotechnics as it's hydrophilic and when even slightly wet it's barely usable. In this application it's better to use potassium chlorate.
blasting it
After a week, i came to conclusion that it'd be best to just blast it without worrying about things like salt dissolved in solution.
I've spend 4 hours slowly boiling it away on low flame.


This resulted in a rock that has 230C.


After cooling it was crushed with a crowbar, crushed in mortar and sieved (visible lumps are not hard, the overall consistency is a bit coarser than a flour).



This cycle would continue many times, until i've hammered my mortar into ground so much that i couldn't pull it out and had to dig around it with a shovel. This has motivated me into breaking only large chunks in mortar and smaller in coffee grinder. This has worked nicely and resulting consistency was of a flour.

This lead to problems with sieving as powder was too fine. It clumped slightly on sieve from absorbing water in air making sieving harder.
And there was a lot of cleaning.


I've barely fit it into 1L bottle, and powder with bottle weighted 1192.8g

I've made a quick sucrose test that i obviously forgot to record, but it had worked and next day i've made another one.

I've used common sugar that was quite coarse so the flame isn't very quick.
what happened?
1760.36g of salt was overall added and product is 1030g? Well accounting for lost sample it could be 1200g still its less than put in.
Maybe sludge at the bottom was actually mostly salt that i didn't dissolve, or a lot of salt creeped out.
Thinking about previous runs, this one was the biggest by 1.5 litre, it run the longest of them with highest current. The difference was the size of cathodes which by far were the largest, also there was negligible amount of magnesium hydroxide deposition on them. It's possible that they reduced most of my product.
Another possibility is that i've decomposed chlorate when drying since decomposition temperature is only 300C. Yet there seemed to be no gas escaping, especially oxygen which would be visible as it was surrounded by flames so i think of it as not very likely.
I can make excuses, but the fact is that compared to all my previous less "professional" runs it's seriously the lowest yield.
If you rightfully see me as incompetent here is a video of someone explaining this reaction in depth https://youtu.be/qSyj03R_d4Q?t=701.